The Overview
In the pharmaceutical industry, timing is everything, and information delay can mean missed opportunities worth millions. With over 430,000 clinical trials registered globally and new data emerging daily, one global pharma organization needed to rethink how it tracked drug development, competitor activity, and licensing opportunities.
Creatyfit Consulting was brought in to design and deliver a real-time pharmacovigilance and clinical trial intelligence platform. Our goal was simple – enable R&D, regulatory, and business teams to act on data before the competition, turning clinical visibility into a strategic advantage.

Client Context & Challenges
Industry: Pharmaceutical / Life Sciences
Use Case: Clinical Trial Monitoring, Competitive Intelligence, R&D Strategy
Initial State: Fragmented manual tracking, slow reporting, and lack of data visibility

Key Challenges
- Manual tracking of thousands of global trials across clinicaltrials.gov and other sources
- Long lead times for report generation (up to 3 days)
- No integrated views across phases, sponsors, or therapeutic areas
- Limited ability to act on licensing, regulatory, or competitive signals in real time
The organization needed a scalable, intelligence-led solution to convert data overload into business-ready insight.
Our Approach
Engineering decision intelligence for pharma leadership.
Our team led the engagement with a consultative, insight-driven approach focused on real-world decision-making needs.
Stakeholder Workshops
We collaborated with R&D leaders, clinical affairs, and regulatory teams to define critical use cases—such as trial prioritization, competitive benchmarking, and in-licensing triggers.
Technology Discovery
We conducted a feasibility scan across data sources and architectures—evaluating ETL pipelines, integration models, and reporting frameworks that could support real-time sync and visual insights.
Prototyping & Validation
Within six weeks, we launched a functional dashboard, validated through rapid user testing cycles.
Agile Implementation
Using a weekly sprint model, we prioritized adaptability and continuous feedback—ensuring the platform grew in lockstep with business needs.
We weren’t just building a dashboard, we were operationalizing strategic foresight.
The Solution
Pharmacovigilance Intelligence Platform
A custom-built, web-based platform designed to deliver fast, actionable insights across the global clinical trial landscape.
Key Features & Functional Capabilities :
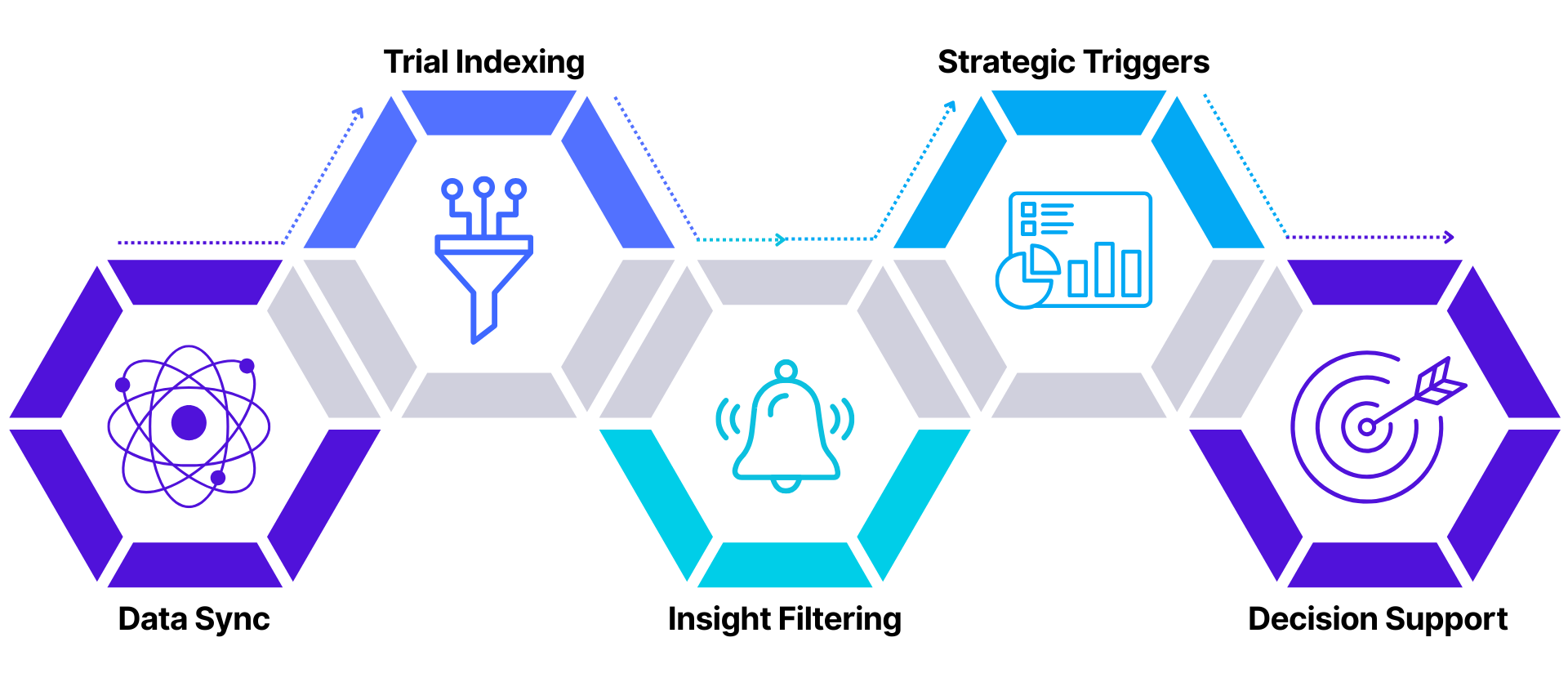
Real-Time Trial Data Integration
- Automated ingestion from clinicaltrials.gov via scheduled ETL pipelines
- Indexed 350,000+ trials at launch with ongoing updates
- Cross-source harmonization for consistent insight
Smart Filtering & Custom Reporting
- Searchable by molecule, condition, sponsor, phase, and geography
- Role-based views and exportable dashboards for R&D, regulatory, and business users
Interactive Pipeline Tracker
- Visualized progress bars by therapeutic area
- Comparative timelines of competitor activity across trial phases
- Color-coded risk and readiness indicator
Strategic Alerts & Triggers
- Custom alerting for trial initiation, phase transitions, or sponsor actions
- Built-in logic for licensing, partnership, or go/no-go decision points
Designed for Speed & Simplicity
- Drag-and-drop report builder for executive teams
- Real-time updates with no manual processing
- Cloud-hosted, secure, and fully scalable
From Data to Decision: Clinical Trial Intelligence Lifecycle
The Impact & Outcomes

80% Reduction in Manual Monitoring Time
Teams moved from spreadsheet tracking to real-time dashboards across global trials.

3 Day Reports Now Generated in 30 Minutes
Faster insight delivery enabled quicker strategy alignment.

9 New Licensing Opportunities Identified
The platform flagged promising molecules aligned with therapeutic focus areas.

Accelerated Competitive Response
Alerts on trial status changes improved agility in responding to competitor moves.

Shift from Reactive to Proactive Clinical Strategy
Teams now operate with foresight, positioning the company as an early mover in key innovation areas.
Want to transform data into strategic action?
Let’s design intelligent platforms that move your business ahead of the curve.
Accelerating R&D Strategy with Clinical Trial Intelligence Automation
A global pharmaceutical company’s partnership with Creatyfit Consulting to develop a real-time clinical trial intelligence platform. The solution transformed how R&D and regulatory teams monitor global trials, reducing manual effort, improving competitive response, and accelerating strategic decision-making.